PIPELINE
Protect Animal Health Market Position:
Creating human-like care for the pet market
Animal drug development takes around 3-6 years to complete at only a fraction of a cost compared to the 12-15 years of costly process for Human Drugs. Protect minimize pipeline risk by first identifying high demand pet disease areas and developing solutions based on proven success in the human market. Our first pipeline candidate, PT-001 addresses the $11 billion pet cancer market using PD-L1 and CTLA-4 immune checkpoint inhibitor. The underlying mechanism has proven to work for multiple cancer types and protect is developing a novel canine therapeutic vaccine to make the advanced treatment option more accessible for pet owners. Currently, Protect is completing the pilot study for PT-001 and will file for INAD in 2024.

Pipeline expansion through acquisition and in-licensing
An incubator for pet healthcare solutions
Beyond the PT-001 vaccine, Protect expands product offering through globally sourcing promising technologies. We act as an incubator and agent for innovative technologies to capitalize on the pet economy boom, particularly in the pet healthcare sector. Protect has 4 core technology platform that help us and our global partner scale drug developments and accelerate product offers to our target patients.

Long-term goal
Constructing a pet medical care ecosystem
Become a global leader in animal healthcare; build coverage across the entire pet healthcare cycle – from diagnostics, predictive health analytics, health maintenance, nutraceutical supplements, to drugs– we are streamlining the pet-care eco-system.

Pet medication covers multiple areas
Comprehensive care of pet medical medications, dosage forms, and screenings
Protect Animal Health goals include not only drugs, but also expansion into pet medical treatments such as dosage forms and screening and multiple pet diseases, including cancer, orthopedics, skin, kidney, infection and heart disease, etc. It hopes to become a pioneer in the field of new animal drugs and become a A cutting-edge new pet drug research and development company in Asia and even the world.

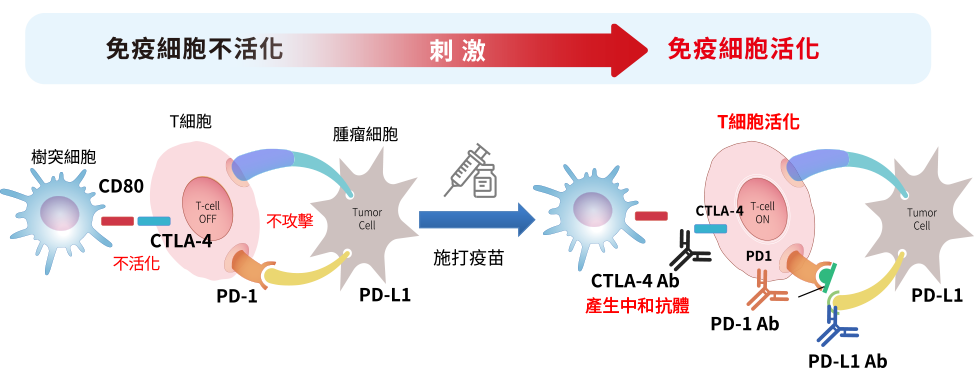
免疫檢查點治療
免疫檢查點治療是一種新興的癌症治療方法,目前已經被證明對許多類型的癌症具有顯著的療效。免疫檢查點是一種能夠調節免疫反應的分子,它們在人體中起著重要的作用,幫助維持免疫系統的平衡。癌症細胞可以利用這些免疫檢查點來逃避免疫系統的攻擊。而治療性疫苗是一種能夠刺激人體免疫系統對癌細胞進行攻擊的疫苗。它們與普通疫苗不同,普通疫苗是用於預防疾病的,而治療性疫苗則是用於治療已經存在的疾病。因此寶泰生醫針對已知且成功的免疫檢查點靶位,開發系列產品線如下:

免疫檢查點治療性疫苗可以作為一種能夠同時針對免疫檢查點和癌細胞的治療方法,它可以刺激免疫系統對癌細胞進行攻擊,並通過調節免疫反應來增強治療效果。
PT001
廣效癌症治療性疫苗 (PD-L1 + CTLA-4)
PT001為次蛋白癌症治療性疫苗,應用目前人類治療癌症最主流的免疫檢查點機制,透過喚醒沉睡的免疫細胞,使免疫系統活化並辨認出腫瘤細胞,運用自身免疫系統達成治療癌症的目的,治療機制如下圖所示:
- 給藥方式:皮下注射,每針0.5 ml。
- 給藥頻率:每兩週給藥一次,總共給藥三次。
- 其他事項:給藥時不須麻醉,無需額外醫療照護。
- 適應症:包含但不限於黑色素瘤、淋巴癌、肥大細胞瘤等多種癌別臨床測試中。
PT002
DNA廣效癌症治療性疫苗(PD-L1 + CTLA-4)
PT002為DNA核糖核酸治療性疫苗,應用目前人類治療癌症最主流的免疫檢查點機制,透過喚醒沉睡的免疫細胞,使免疫系統活化並辨認出腫瘤細胞,運用自身免疫系統達成治療癌症的目的。DNA疫苗具有無佐劑、常溫可保存等優點,應用無針式基因槍給藥,提供皮內注射和快速無痛的給藥方式,目標解決寵物貓咪特有的注射部位腫瘤問題,同時達成治療癌症的目的。

- 給藥方式:皮內注射,每針0.1 ml。
- 給藥頻率:每兩週給藥一次,總共給藥三次。
- 其他事項:給藥時不須麻醉,無需額外醫療照護。
- 適應症:包含但不限於黑色素瘤、淋巴癌、肥大細胞瘤等多種癌別臨床規劃中。